electron dot structure|Iba pa : Tuguegarao Lewis structures – also called Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDs) – are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any covalently bonded molecule, as well as coordination compounds. The Lewis structure wa. Starbucks Abreeza Mall, Davao City: See 33 unbiased reviews of Starbucks Abreeza Mall, rated 3.5 of 5 on Tripadvisor.? bsd 418? bungo stray dogs 1218? bungou stray dogs 369; Character? dazai osamu 727? osamu dazai 314? sigma (bungo stray dogs) 55; Artist? queersturbates 15; General? 1boy 1630982? 1cuntboy 6417? bellybutton piercing 42? blush 2235138? bondage 315062? brown hair 778814? cunnilingus 85229? cuntboy 43326? .
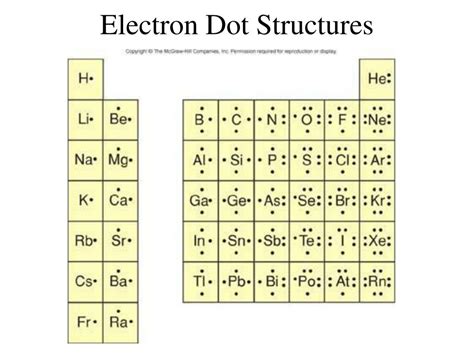
PH0 · lewis dot structure chart
PH1 · electron dot structure questions class 10
PH2 · electron dot structure of nh3
PH3 · electron dot structure for cl
PH4 · electron dot structure for chlorine
PH5 · electron dot structure for carbon
PH6 · electron dot structure for aluminum
PH7 · electron dot structure calculator
PH8 · Iba pa
To set up new Spectrum services, including Spectrum Internet, cable TV or Home Phone plan, call Spectrum Customer Service at 855.860.9068, Monday-Sunday, 7AM-2AM ET. If you have account and billing questions, contact customer service 24 x 7 at 833.949.0036 For 24 x 7 technical support and online assistance with your account, please visit .Quezon City, one of the country's most densely populated urban centers, is asking residents to register for a single ID whch can be used to access health, transportation, and other social services.. The QCitizen ID project mirrors a national government effort to establish a single database that can be used to access almost .
electron dot structure*******Learn how to draw Lewis dot structures or electron dot structures to represent the bonding and lone pairs of atoms in molecules. See examples, videos and FAQs on Lewis structures and their .
Learn how to draw Lewis electron dot diagrams for atoms and ions using dots around the symbol of the element. See examples, exercises and explanations for .Lewis structures – also called Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDs) – are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any covalently bonded molecule, as well as coordination compounds. The Lewis structure wa.
Learn how to write Lewis symbols for neutral atoms and ions using dots to represent valence electrons. See examples, exercises and key takeaways for this topic. Learn how to draw Lewis structures, also known as electron dot structures, for covalent molecules and coordination compounds. Follow the steps to pick a central .Learn how to draw Lewis electron dot diagrams for atoms and monatomic ions with less than 20 atomic number. See examples of covalent bonding and Lewis structures for .
Learn how to draw Lewis dot structures for molecules and ions using the octet rule and electronegativity. Watch the video and read the comments and questions from other .
A Lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Shared pairs of electrons are drawn as lines between atoms, while lone pairs .Learn how to use Lewis dot symbols to describe covalent bonding, stoichiometry, and physical properties of compounds. Follow the easy method procedure to construct Lewis .
Learn how to draw and interpret Lewis electron-dot structures to show the bonding in covalent molecules. See examples of hydrogen, cholesterol, and other .The structure on the right is the Lewis electron structure, or Lewis structure, for H 2 O. With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. . The unpaired electron is usually placed in the Lewis Dot Structure so that each element in the structure will have the lowest formal charge possible.electron dot structure Iba paA Lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. When constructing a Lewis diagram, keep in mind the octet rule, which refers to the tendency of atoms to gain, lose, or share .
A Lewis dot structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side.The former, known as a ‘Lewis dot diagram,’ indicates a pair of shared electrons between the atomic symbols, while the latter, known as a ‘Lewis structure,’ uses a dash to indicate the pair of shared electrons that form a covalent bond. More complicated molecules are depicted this way as well. Lewis structures, also known as electron dot structures, are named after Gilbert N. Lewis, who described them in a 1916 article titled, "The Atom and the Molecule." Lewis structures depict the bonds between atoms of a molecule, as well as any unbonded electron pairs. You can draw a Lewis dot structure for any covalent molecule or .
Iba paWe draw the dot structure in the exact same manner, and then calculate the formal charges for the atoms in the molecule. Remember that formal charge is calculated by taking the # of valence electrons, minus the lone electrons and the bonds, and we show that charge next to the molecule.Take ::O=C=O:: for example.
Follow these simple steps to draw Lewis dot structures: Draw the atoms on paper and put dots around them to represent valence electrons of the atom. Be sure to have the correct number of electrons. If the species is an ion, add or subtract electrons corresponding to the charge of the ion. Add an electron for every negative (-) charge, .electron dot structure The structure in the figure above is much more helpful – we see how the different atoms are connected together to form the molecule. Lewis Electron-Dot Structures. In a previous chapter, you learned that the valence electrons of an atom can be shown in a simple way with an electron dot diagram. A hydrogen atom is shown as H• .Gilbert N. Lewis introduced a diagrammatic system to represent the valence shell electrons in an atom. It helps us a lot while doing chemical bonding. He used dots to represent the valence electrons. He used lines to indicate bond between two electrons. Here's an example of carbon, with its valence electrons using Lewis dot structure. Gilbert Lewis .
Figure 3.1.1 3.1. 1: Lewis dot structure of atoms comprising ammonia and the dot structure of the molecule. (Kathryn Haas; CC-NC-BY-SA) The drawing of Lewis electron-dot structures is guided largely by the octet rule: that atoms form bonds to achieve eight electrons in their valence shell. For many elements, a full valence shell has an .In 1916, American chemist, Gilbert N. Lewis, introduced bond lines to electron dot structures. These structures, also known as lewis structures or electron dot structures, are drawings that visually demonstrate how .
Here are the steps to draw a Lewis structure. The example is for the nitrate ion. A Lewis structure is a diagram that shows the chemical bonds between atoms in a molecule and the valence . Each dot in the structure represents one valence electron in the compound. For example, \(\ce{H_2}\) could be drawn as \(\ce{H} : \ce{H}\). Each dot represents one valence electron, and the fact that they are placed between the two atoms means that they are being shared bas a covalent bond. For larger molecules, it can .

Finally, you'll understand all those weird pictures of molecules with the letters and the lines and the dots! Those are lewis dot structures. Let's learn how.So when I look at my dot structure, I can check to make sure I have the correct number of valence electrons. I need 14. So let's go ahead and count them. So this would be two here, four, six, eight, 10, 12 and 14. So I have the correct number of valence electrons represented in my dot structure. I also have an octet of electrons around my carbons.
What is Lewis Structure. Lewis structure, also known as Lewis dot structure or electron dot structure, is a simple and straightforward way of representing the outermost electron shell in a chemical species like an atom, ion, or molecule. It shows how electrons are positioned around the atoms either as lone pairs or in a chemical bond, typically . Figure 3.1.1 3.1. 1: Lewis dot structure of atoms comprising ammonia and the dot structure of the molecule. (Kathryn Haas; CC-NC-BY-SA) The drawing of Lewis electron-dot structures is guided largely by the octet rule: that atoms form bonds to achieve eight electrons in their valence shell. For many elements, a full valence shell has an .A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the .
Mega Moolah (Microgaming): Overview. If you are an online slot gamer and you haven’t heard of Mega Moolah already, then clearly, you're out of the loop. This offering from Microgaming is one of, if not the, most high-profile slots from the developer.This is not least due to the fact that it incorporates four different progressive jackpots, but also because .
electron dot structure|Iba pa